UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
|
|
|
|
|
|
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer |
|
|
|
|
|
|
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Trading Symbol(s) |
Name of Each Exchange on Which Registered |
|
|
|
|
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
|
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
|
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
|
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
|
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 8.01 Other Events
On March 24, 2020, Allakos Inc. (the “Company”) hosted a conference call and webcast to present a corporate update. A copy of the presentation is filed as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit Number |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
1
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Allakos Inc. |
|
|
|
|
|
|
|
Date: March 24, 2020 |
|
By: |
/s/ Robert Alexander |
|
|
|
|
Robert Alexander |
|
|
|
|
Chief Executive Officer |
2

Developing Therapeutic Antibodies Targeting Allergic, Inflammatory and Proliferative Diseases Corporate Update March 2020 Exhibit 99.1

Disclaimer This presentation contains forward-looking statements. All statements other than statements of historical fact contained in this presentation, including statements regarding the financial position of Allakos Inc. (“Allakos,” the “Company,” “we” or “our”); the generation of future value; business strategy; plans and objectives for future operations; our expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates; our expectations with regard to the initiation, design, timing and results of our clinical studies, preclinical studies and research and development programs, including the timing and availability of data from such studies; our preclinical, clinical and regulatory development plans for our product candidates, including the timing or likelihood of regulatory filings and approvals for our product candidates; the size of the market opportunity for our product candidates in the diseases we are targeting; and our expectations with regard to our ability to acquire, discover and develop additional product candidates and advance such product candidates into, and successfully complete, clinical studies, are forward-looking statements. Allakos has based these forward-looking statements on its estimates and assumptions and its current expectations and projections about future events. The words ‘‘anticipate,’’ ‘‘believe,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘target,’’ ‘‘should,’’ ‘‘would,’’ and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this presentation speak only as of the date of this presentation and are subject to a number of risks, uncertainties, and assumptions, including, but not limited to: the Company’s early stages of clinical drug development; the Company’s ability to timely complete clinical trials for, and if approved, commercialize antolimab (AK002), its lead compound; the Company’s ability to obtain required regulatory approvals for its product candidates; uncertainties related to the enrollment of patients in its clinical trials; the Company’s ability to demonstrate sufficient safety and efficacy of its product candidates in its clinical trials; uncertainties related to the success of later-stage clinical trials, regardless of the outcomes of preclinical testing and early-stage trials; market acceptance of the Company’s product candidates; uncertainties related to the projections of the size of patient populations suffering from some of the diseases the Company is targeting; the Company’s ability to advance additional product candidates beyond AK002; the Company’s ability to obtain additional capital to finance its operations; and other risks described in the “Risk Factors” section included in our periodic filings that we have made and will make with the Securities and Exchange Commission (“SEC”). In addition, Allakos operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for Allakos’s management to predict all risks, nor can Allakos assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements that Allakos may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Allakos does not undertake any obligation to update or revise any forward-looking statements, to conform these statements to actual results or to make changes in Allakos’ expectations, except as required by law. Accuracy of Data: This presentation contains statistical data based on independent industry publications or other publicly available information, as well as other information based on Allakos’s internal sources. We have not independently verified the accuracy or completeness of the data contained in these industry publications and other publicly available information. Accordingly, Allakos makes no representations as to the accuracy or completeness of that data. Additional Information:The Company has filed and will file Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, and Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about the Company. You may get these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated.

Agenda Robert Alexander, PhD Overview 5:00 – 5:20 PM Henrik Rasmussen, MD PhD Initiated clinical studies MGID Phase 1 study results 5:20 – 5:50 PM Q&A 5:50 – 6:00 PM

Overview Robert Alexander, PhD CEO – Allakos

Executive Summary Recently initiated Phase 3 study in Eosinophilic Gastritis (EG) and/or Eosinophilic Duodenitis (EoD; previously referred to as EGE) Recently initiated Phase 2/3 study in Eosinophilic Esophagitis (EoE) First group of subjects dosed in Phase 1 subcutaneous healthy volunteer study Positive results from Phase 1 open-label study in Mast Cell GI Disease (MGID) EGID & MGID prevalence study in patients with chronic functional GI disease is underway

Impact of Coronavirus on Allakos Clinical Trials Phase 3 EG/EoD and Phase 2/3 EoE sites have been activated and potential patients have been identified To avoid study disruption, we have decided to delay enrollment in the Phase 3 EG/EoD and the Phase 2/3 EoE studies Current estimated completion of H2 2021 but may be delayed Subcutaneous and Prevalence studies currently continue to enroll Current estimated completion of H2 2020 but may be delayed We will continue to carefully monitor the situation to minimize the impact on our development programs
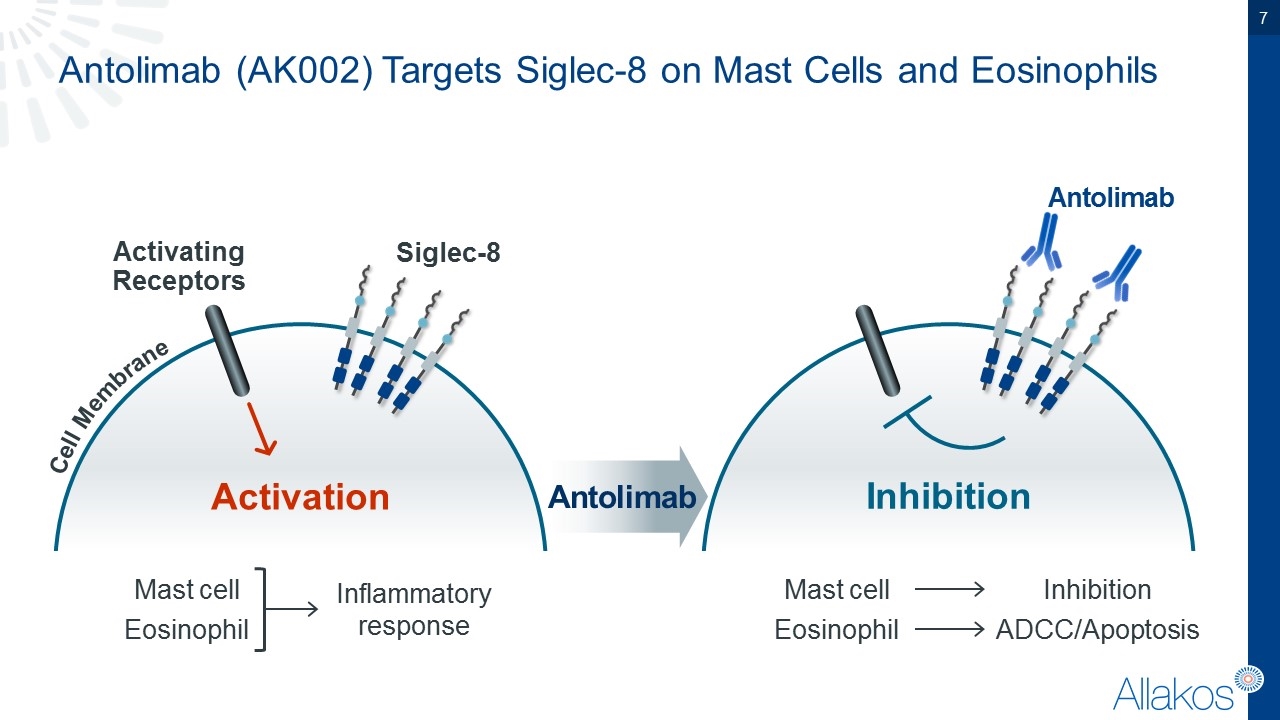
Antolimab (AK002) Targets Siglec-8 on Mast Cells and Eosinophils Cell Membrane Activating Receptors Activation Siglec-8 Mast cell Eosinophil Inflammatory response Antolimab Inhibition Mast cell Eosinophil Inhibition ADCC/Apoptosis Antolimab

Mast Cells and Eosinophils Are Key Drivers of Inflammatory Disease

Eosinophils and Mast Cells Play a Significant Role in Many Diseases Mast Cell Activation Syndrome Indolent Systemic Mastocytosis Chronic Urticaria Atopic Dermatitis Eosinophilic Gastritis Eosinophilic Esophagitis Eosinophilic Duodenitis Ulcerative Colitis Vernal Keratoconjunctivitis GASTROINTESTINAL DISEASES OPHTHALMIC DISEASES RESPIRATORY DISEASES SKIN DISEASES MULTI-ORGAN DISEASES Atopic Keratoconjunctivitis Asthma Perennial Allergic Conjunctivitis Eosinophilic Asthma Idiopathic Pulmonary Fibrosis Irritable Bowel Syndrome Crohn’s Disease

Eosinophilic Gastrointestinal Diseases (EGIDs) ESOPHAGUS STOMACH DUODENUM Eosinophilic Gastritis (EG) Eosinophilic Esophagitis (EoE) Eosinophilic Duodenitis (EoD) = US Prevalence ~20-25K ~25K ~200K EG, EoD, EoE Chronic Eosinophilic Inflammation of the Stomach, Duodenum, or Esophagus Eosinophils and mast cells are important drivers of disease Symptoms: abdominal pain, nausea, early satiety, loss of appetite, bloating, abdominal cramping, vomiting, diarrhea, and dysphagia No FDA-approved treatment for EG, EoD, or EoE Current standard of care: diet and/or steroids Large market opportunity

EGID Biopsies Have Elevated Eosinophils & Mast Cells Source: Youngblood B, et al. JCI Insights. 2019 Eosinophils 10 8 6 4 2 0 Mast Cells 15 10 5 0 Mast Cells 12 8 4 0 Eosinophils 12 8 4 0 % of CD45+ Viable Cells Esophagus Stomach Normal EoE Normal EoE Normal EG Normal EG *p<0.05

Eos and Mast Cells Are Activated in EGID Biopsies CD63 3000 2000 1000 0 150000 100000 50000 0 IgE 8000 4000 2000 0 6000 CD49d 60000 40000 20000 0 CD11b Eosinophils Mast Cells Normal EG Normal EG Normal EG Normal EG Source: Youngblood B, et al. JCI Insights. 2019 *p<0.05 Eosinophils and mast cells both appear to play a pathogenic role Expression (MFI)

End of Phase 2 (ENIGMA) Meeting Summary

Key Takeaways Histologic Co-Primary Endpoint Consistent with EoE guidance, FDA recommended using a responder analysis Histologic response thresholds set at ≤4 eos/hpf in the stomach and/or ≤15 eos/hpf in the duodenum Symptom Co-Primary Endpoint The same PRO questionnaire will be used in Phase 3 as was used in ENIGMA FDA recommended using a Total Symptom Score consisting of the 6 most frequent and severe symptoms (TSS-6): abdominal pain, nausea, bloating, early satiety, abdominal cramping, loss of appetite; vomiting and diarrhea are measured but excluded from the co-primary endpoint Duration of study Consistent with EoE guidance, FDA recommended a 6-month study Change in nomenclature Eosinophilic Gastroenteritis is now referred to as Eosinophilic Duodenitis

Phase 3 Eosinophilic Gastritis and/or Eosinophilic Duodenitis (formally referred to as EGE) Study Design Henrik S. Rasmussen, MD PhD Chief Medical Officer – Allakos

Phase 3 Eosinophilic Gastritis (EG) and/or Duodenitis (EoD) Study Study Design Multi-center, randomized, double-blind, placebo-controlled study in EG/EoD Adult patients with active moderate to severe symptoms Biopsy confirmed EG/EoD Stomach: ≥30 eos/high powered field (hpf) in 5 hpfs, and/or Duodenum: ≥30 eos/hpf in 3 hpfs 160 Patients – 2 arms 80 patients 1.0, 3.0, 3.0, 3.0, 3.0, 3.0 mg/kg antolimab 80 patients placebo 6 monthly doses Same patient population as Phase 2 ENIGMA study
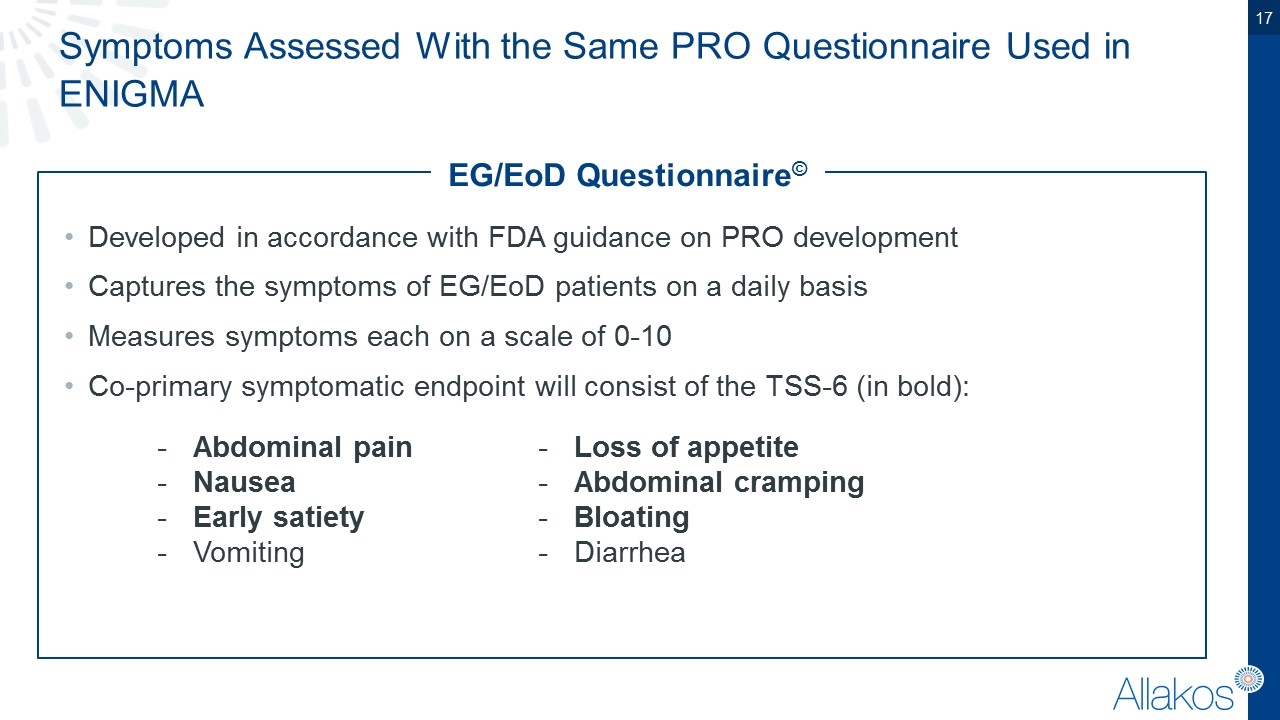
Symptoms Assessed With the Same PRO Questionnaire Used in ENIGMA Abdominal pain Nausea Early satiety Vomiting Loss of appetite Abdominal cramping Bloating Diarrhea Developed in accordance with FDA guidance on PRO development Captures the symptoms of EG/EoD patients on a daily basis Measures symptoms each on a scale of 0-10 Co-primary symptomatic endpoint will consist of the TSS-6 (in bold): EG/EoD Questionnaire©

Phase 3 EG/EoD Endpoints Histologic Co-Primary Endpoint Proportion of responders: Stomach: ≤4 eos/hpf in 5 hpfs, and/or Duodenum: ≤15 eos/hpf in 3 hpfs Symptom Co-Primary Endpoint Absolute change in patient reported TSS-6 Key Secondary Endpoints Percent change in tissue eosinophils Treatment responders: patients who achieve tissue eosinophil thresholds AND >30% improvement in TSS-6 Phase 3 study has >90% power

Phase 2 ENIGMA Results Analyzed Against Phase 3 Endpoints (ITT) Co-Primary Endpoints Antolimab Placebo p – value 3 mg/kg Histologic Endpoint1 Proportion of Responders 95% 0% <0.0001 Symptom Endpoint2 Mean Absolute Change in TSS-6 -16.6 -8.1 0.0162 Mean Percent Change in TSS-6 -59% -27% 0.0033 1Responder: Patients who achieve ≤4 eos/hpf in 5 hpfs in the stomach and/or ≤15 eos/hpf in 3 hpfs in the duodenum 2TSS-6: Total score of 6 symptoms (abdominal pain, nausea, bloating, early satiety, abdominal cramping, and loss of appetite)

Phase 2/3 Eosinophilic Esophagitis (EoE) Study Design

Phase 2/3 Eosinophilic Esophagitis (EoE) Study Study Design Multi-center, randomized, double-blind, placebo-controlled study in Eosinophilic Esophagitis Patients (12 to 80 years old) with active moderate to severe symptoms Biopsy confirmed EoE Esophagus: ≥15 eos in 1 hpf 300 Patients – 3 arms 100 patients 1.0, 3.0, 3.0, 3.0, 3.0, 3.0 mg/kg antolimab 100 patients 1.0, 1.0, 1.0, 1.0, 1.0, 1.0 mg/kg antolimab 100 patients placebo 6 monthly doses

Phase 2/3 Eosinophilic Esophagitis Endpoints Histologic Co-Primary Endpoint Proportion of responders: ≤6 eos/hpf in 1 hpf in the esophagus Symptom Co-Primary Endpoint Absolute change in patient reported Dysphagia Symptom Questionnaire (DSQ) Key Secondary Endpoints Percent change in esophageal tissue eosinophil count Percent change in DSQ score Follows 2019 FDA EoE Guidance

Phase 1 Subcutaneous Healthy Volunteer Study
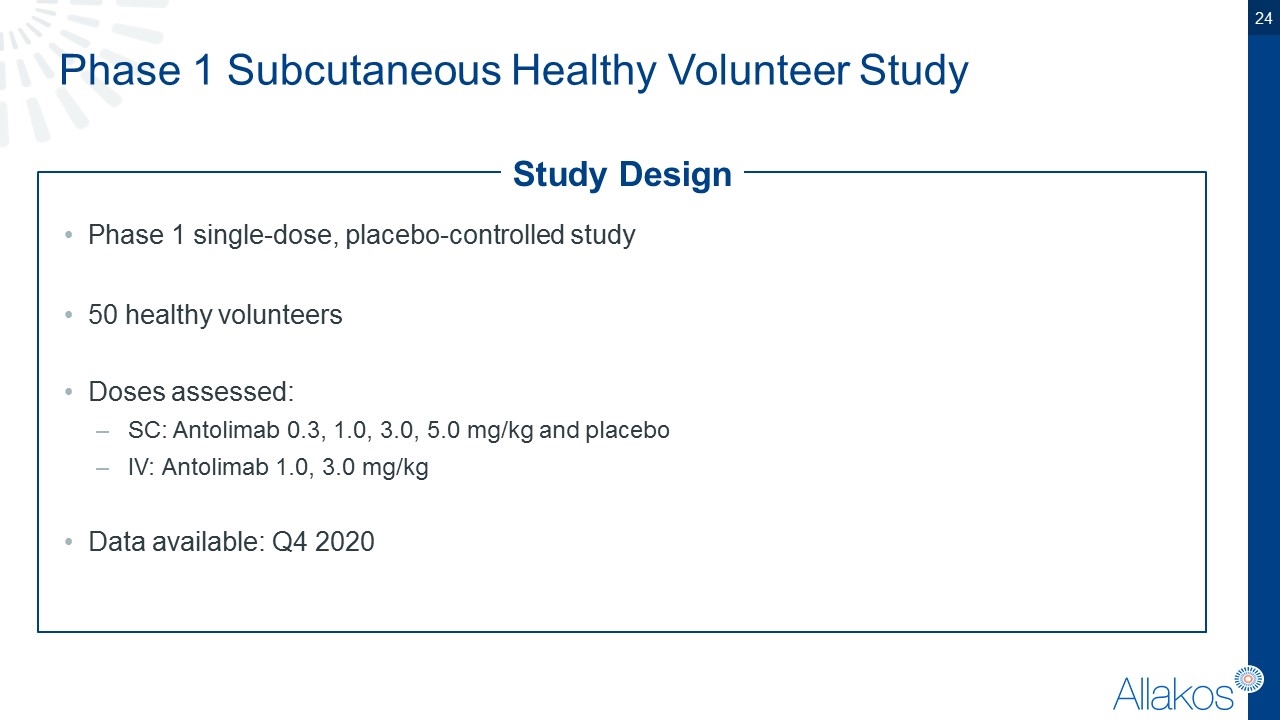
Phase 1 Subcutaneous Healthy Volunteer Study Study Design Phase 1 single-dose, placebo-controlled study 50 healthy volunteers Doses assessed: SC: Antolimab 0.3, 1.0, 3.0, 5.0 mg/kg and placebo IV: Antolimab 1.0, 3.0 mg/kg Data available: Q4 2020

Mast Cell Gastrointestinal Disease (MGID) Patients with Chronic GI Symptoms and Elevated Mast Cells

ENIGMA Screening Patient Distribution Met Symptom Criteria n=88 Entered Screening n=113 16 of 88 symptomatic patients had elevated mast cells only EG / EoD n=72 Mast Cell GI Disease n=16 MGID: ≥30 Mast Cells EG/EoD: ≥30 Eos & Mast Cells1 165 met all enrollment criteria for ENIGMA; 1 patient had elevated eos only

Phase 1 Mast Cell GI Disease (MGID) Study Study Design Multi-center, open-label, multi-dose, Phase 1 study Active moderate to severe symptoms as measured by PRO used in ENIGMA N=7 patients Biopsy confirmed elevated mast cells Stomach: ≥30 mast cells/high powered field (hpf) in 5 hpfs, and/or Duodenum: ≥30 mast cells/hpf in 3 hpfs 6 monthly doses 0.3, 1.0, 3.0, 3.0, 3.0, 3.0 mg/kg antolimab

Baseline Gastrointestinal Eosinophils and Mast Cell Counts Mean Gastrointestinal Cells Eosinophils Mast Cells Mean Gastrointestinal Cells Eosinophils Mast Cells ENIGMA EG / EoD (n=65) MGID (n=7)

EG/EoD and MGID Patients Have Similar Symptomatic Burden Symptom Reported Symptom Intensity During Screening (0-10) Mean Early Satiety Bloating Abdominal Pain Abdominal Cramping Loss of Appetite Nausea Diarrhea Vomiting EG/EoD patients (n=65) MGID Patients (n=7)

64% Improvement in Total Symptom Score Mean % Change from BL Mean % Change from BL Antolimab 3.0 mg/kg Placebo MGID EG / EoD in ENIGMA PRO TSS-8: Change from Baseline Antolimab 3.0 mg/kg

Improvement Across All Symptoms Measured MGID: PRO Symptom Score Antolimab (n=7) Median Score -69% -78% -100% -90% -91% -57% -91% -85% Baseline End of Tx
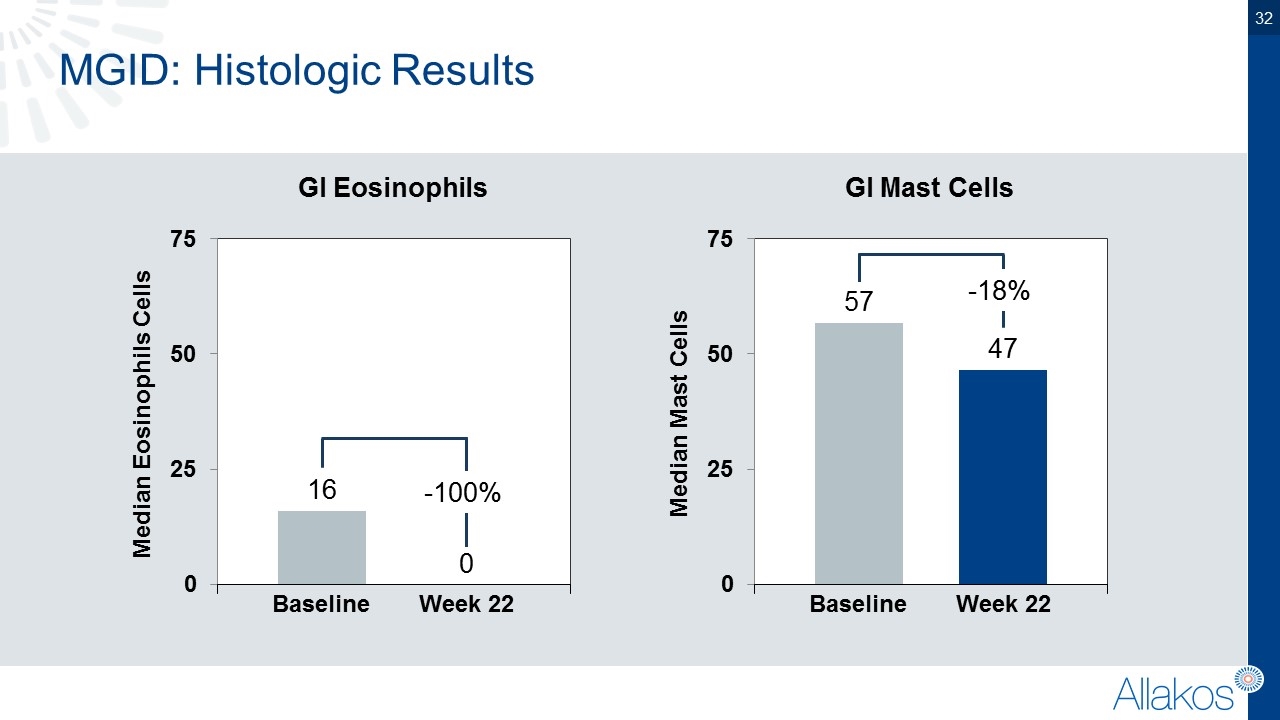
MGID: Histologic Results Median Eosinophils Cells Baseline Week 22 Median Mast Cells Baseline Week 22 -100% GI Eosinophils GI Mast Cells -18%

MGID: Safety Summary Generally well tolerated Most common AE was infusion related reactions (IRR), all of which were mild No drug-related SAEs

EGID & MGID Prevalence Study

EGID & MGID Prevalence Study Study Design Multi-center study to assess prevalence of EGIDs and MGID in patients with chronic functional GI symptoms with or without diagnosis of Irritable Bowel Syndrome (IBS), Functional Dyspepsia (FD), or Chronic Gastritis Biopsies from >200 adult patients with active moderate to severe symptoms as assessed by PRO questionnaire used in ENIGMA and Phase 3 EG/EoD study Primary endpoint: Proportion of patients with EG, EoD, or MGID Study initiated late January, data expected H2 2020

Summary Recently initiated Phase 3 EG/EoD and Phase 2/3 EoE studies First group dosed in subcutaneous healthy volunteer study Antolimab improves symptoms in patients with chronic GI symptoms and elevated mast cells (MGIDs) EGID & MGID prevalence study in patients with chronic functional GI disease is well underway

Corporate Updates

Strong Balance Sheet and Significant IP Protection Cash, Cash Equivalents and Investments in Marketable Securities as of Dec 31, 2019 $495.9M Q4 2019 Operating Expenses $26.9M Antolimab US patents first to expire 2035 Lonza currently manufactures antolimab

Anticipated Near-term Milestones H2’20 EGID & MGID Prevalence Study Results H2’21 Phase 2/3 EoE Results* H2’20 Phase 1 Data For Subcutaneous Antolimab Q2’20 Phase 2 Extension Data in EG and/or EoD H2’21 Phase 3 EG and/or EoD Results* *Estimates subject to delay due to coronavirus

Thank you Q&A
