Allakos Announces Positive Phase 2 Results for AK002 in Patients with Xolair Refractory Chronic Spontaneous Urticaria and Provides Additional Data from Chronic Urticaria Study Cohorts
-- 55% (6/11) response rate by UCT in Xolair Refractory CSU patients --
-- 49% reduction in UAS7 in Xolair Refractory CSU patients --
-- 77% (10/13) complete hive response by HSS7 in Xolair Naïve CSU patients --
-- 54% (7/13) complete itch response by ISS7 in Xolair Naïve CSU patients --
-- 54% (7/13) complete response by UAS7 in Xolair Naïve CSU patients --
-- 100% (7/7) complete response in PCE test in Cholinergic Urticaria patients --
-- 50% response in Fric Test in Symptomatic Dermographism patients --
“The results seen in the Xolair failure cohort are remarkable,” said principal investigator of the study, Dr.
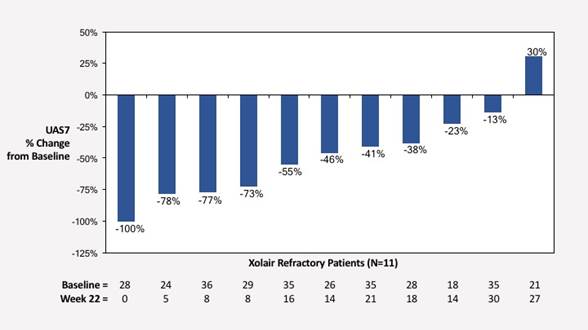
The Xolair failure cohort enrolled 11 patients who failed to have an adequate response to prior Xolair treatment. Patients in this cohort had received an average of 10 months of Xolair treatment at doses as high as 600 mg per month. Patients were required to discontinue Xolair for at least two months before screening but could continue H1 antihistamines at doses of up to four times the labeled dosage throughout the screening period and study. Baseline symptom scores, as measured by Urticaria Control Test (UCT) and Urticaria Activity Score (UAS7)* were collected over the 4-week screening period. Patients with baseline UCT scores of less than 12, indicative of poorly-controlled urticaria, were enrolled in the study and treated with an initial AK002 dose of 0.3 mg/kg at baseline, followed by a dose of 1.0 mg/kg on day 28, and then received monthly doses of either 1.0 or 3.0 mg/kg, depending on response, for up to six doses. The primary efficacy endpoint was change from baseline in UCT assessed at week 22, two weeks after the last dose of AK002.
AK002 was generally well tolerated. The most common adverse event was mild to moderate infusion-related reactions (flushing, feeling of warmth, headache, nausea, and dizziness) which occurred mostly during the first infusion.
Efficacy data from the Xolair failure cohort are presented below; more detailed results from the study will be presented at an upcoming medical conference.
| Xolair Failure Chronic Spontaneous Urticaria Cohort | Baseline | Week 22 |
| Average UCT Score | 3.7 | 8.5 |
| UCT Complete Response | - | 4/11 (36%) |
| UCT Partial Response | - | 2/11 (18%) |
| UCT No Response | - | 5/11 (45%) |
| Average UAS7 | 28.7 | 14.7 (-49%) |
*UCT and UAS7 are established patient reported outcome scales for assessing urticaria control and measuring key symptoms. UCT is a scale between 0 and 16, with higher scores indicating greater urticaria control, and UAS7 is a scale between 0 and 42, with lower scores indicating improvement of symptoms. UCT Complete response was defined as a greater than a 3-point improvement from baseline and a score greater than 12. UCT Partial response was defined as a greater than a 3-point improvement from baseline.
Xolair Failure Chronic Spontaneous Urticaria Cohort: Change from Baseline in UAS7
| Xolair Failure Chronic Spontaneous Urticaria Cohort | Baseline | Week 22 |
| Proportion with UAS7 ≤ 6 | 0% | 2/11 (18%) |
| Proportion with UAS7 = 0 | 0% | 1/11 (9%) |
| Proportion with ISS7 = 0 | 0% | 1/11 (9%) |
| Proportion with HSS7 = 0 | 0% | 1/11 (9%) |
Additional Data from Chronic Urticaria Study Cohort
The Company also released data showing that high proportions of patients receiving AK002 achieved UAS7 = 0, HSS7 = 0, ISS7 = 0 in the Xolair Naïve cohort and that high proportions of patients had negative provocation test results in the cholinergic urticaria and symptomatic dermographism cohorts.
| Xolair Naïve CSU Cohort (N=13) | Baseline | Week 22 |
| Average UCT Score | 3.2 | 14.2 |
| UCT Complete Response | - | 12/13 (92%) |
| UCT Partial Response | - | 0/13 (0%) |
| UCT No Response | - | 1/13 (8%) |
| Average UAS7 Score | 18.5 | 4.6 (-75%) |
| Proportion with UAS7 ≤ 6 | 0% | 8/13 (62%) |
| Proportion with UAS7 = 0 | 0% | 7/13 (54%) |
| Proportion with ISS7 = 0 | 0% | 7/13 (54%) |
| Proportion with HSS7 = 0 | 0% | 10/13 (77%) |
| Cholinergic Urticaria Cohort (N=11) | Baseline | Week 22 |
| Average UCT Score | 5.4 | 11.8 |
| UCT Complete Response | - | 9/11 (82%) |
| UCT Partial Response | - | 0/11 (0%) |
| UCT No Response | - | 2/11 (18%) |
| Pulse Control Ergometry (PCE) Exercise Test Negative1 | 0% | 7/7 (100%) |
| Symptomatic Dermographism Cohort (N=10) | Baseline | Week 22 |
| Average UCT Score | 5.7 | 9.1 |
| UCT Complete Response | - | 4/10 (40%) |
| UCT Partial Response | - | 3/10 (30%) |
| UCT No Response | - | 3/10 (30%) |
| FRIC Test Itch Negative | 0% | 5/10 (50%) |
| FRIC Test Hives Negative (Critical Friction Threshold) | 0% | 4/10 (40%) |
1Seven patients had positive pce tests at entry
About the Phase 2 Chronic Urticaria Study
The open-label Phase 2 study was conducted at four sites in the U.S. and
About Chronic Urticarias
Chronic urticarias are a group of inflammatory skin diseases that are believed to be caused by the inappropriate activation of the mast cells in the skin. Symptoms of urticaria include severe itching, hives, and edema with symptoms lasting for many years. Whereas there is no identified trigger for chronic spontaneous urticaria, other chronic urticarias are caused by triggers such as physical contact with the skin (symptomatic dermographism), or passive or active increases in body temperature (cholinergic urticaria). It has been estimated that 0.5 to 1.0 percent of the U.S. population suffers from a form of chronic urticaria. First-line treatment consists of H1 antihistamine medication, however a significant number of patients do not receive adequate benefit even at four times the labeled dose. Xolair is the only agent approved for antihistamine refractory chronic spontaneous urticaria but is not indicated for other forms of chronic urticaria.
About Allakos
Allakos is a clinical stage biotechnology company developing antibodies that target immunomodulatory receptors present on immune effector cells involved in allergic, inflammatory, and proliferative diseases. The Company’s lead antibody, AK002, targets Siglec-8, an inhibitory receptor selectively expressed on human mast cells and eosinophils. Inappropriately activated eosinophils and mast cells have been identified as key drivers in a number of severe diseases affecting the gastrointestinal tract, eyes, skin, lungs and other organs. AK002 has completed two Phase 1 trials, one in healthy volunteers and a single ascending dose trial in patients with indolent systemic mastocytosis, and one Phase 2 trial in patients with chronic urticaria. AK002 demonstrated pharmacodynamic activity in trials and in the trials involving patients with chronic urticaria and indolent systemic mastocytosis, patients reported improvements in their symptoms. AK002 is being tested in a double-blind, placebo-controlled Phase 2 trial for the treatment of eosinophilic gastritis and eosinophilic gastroenteritis. In addition, Allakos is conducting multiple-dose trials with AK002 in indolent systemic mastocytosis and severe allergic conjunctivitis. For more information, please visit the Company's website at www.allakos.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Such forward-looking statements include, but are not limited to, the timing of top-line results from Allakos’ ongoing clinical trials. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from current expectations and beliefs, including but not limited to: Allakos’ early stages of clinical drug development; Allakos’ ability to timely complete clinical trials for, and if approved, commercialize AK002, its lead compound; Allakos’ ability to obtain required regulatory approvals for its product candidates; uncertainties related to the enrollment of patients in its clinical trials; Allakos’ ability to demonstrate sufficient safety and efficacy of its product candidates in its clinical trials; uncertainties related to the success of later-stage clinical trials, regardless of the outcomes of preclinical testing and early-stage trials; market acceptance of Allakos’ product candidates; uncertainties related to the projections of the size of patient populations suffering from the diseases
Source:
Investor Contact:Adam Tomasi , COO, CFO ir@allakos.com Media Contact:Denise Powell denise@redhousecomms.com
Source: Allakos Inc.