UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
May 7, 2019
Allakos Inc.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
|
|
Delaware |
|
001-38582 |
|
45-4798831 |
|
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(IRS Employer |
975 Island Drive, Suite 201
Redwood City, California 94065
(Address of principal executive offices, including zip code)
(650) 597-5002
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
|
☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
|
☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
|
☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
|
☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
Trading Symbol(s) |
Name of Each Exchange on Which Registered |
|
Common Stock, par value $0.001 |
ALLK |
The Nasdaq Global Select Market |
On May 7, 2019, Allakos Inc. (the “Company”) hosted a conference call and webcast to present detailed results from its Phase 1 trial in Patients with Severe Allergic Conjunctivitis. A copy of the presentation is filed as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
|
Exhibit Number |
|
Description |
|
|
|
|
|
99.1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
Allakos Inc. |
|
|
|
|
|
|
|
Date: May 7, 2019 |
|
By: |
/s/ Robert Alexander |
|
|
|
|
Robert Alexander |
|
|
|
|
President and Chief Executive Officer |

Developing Therapeutic Antibodies Targeting Allergic, Inflammatory and Proliferative Diseases Phase 1b SAC Results May 7, 2019 Exhibit 99.1

Disclaimer This presentation contains forward-looking statements. All statements other than statements of historical fact contained in this presentation, including statements regarding the financial position of Allakos Inc. (“Allakos” or the “Company”); the generation of future value; business strategy; plans and objectives for future operations; our expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates; our expectations with regard to the results of our clinical studies, preclinical studies and research and development programs, including the timing and availability of data from such studies; our preclinical, clinical and regulatory development plans for our product candidates, including the timing or likelihood of regulatory filings and approvals for our product candidates; and our expectations with regard to our ability to acquire, discover and develop additional product candidates and advance such product candidates into, and successfully complete, clinical studies, are forward-looking statements. Allakos has based these forward-looking statements on its estimates and assumptions and its current expectations and projections about future events. The words ‘‘anticipate,’’ ‘‘believe,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘target,’’ ‘‘should,’’ ‘‘would,’’ and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this presentation speak only as of the date of this presentation and are subject to a number of risks, uncertainties, and assumptions, including, but not limited to: the Company’s early stages of clinical drug development; the Company’s ability to timely complete clinical trials for, and if approved, commercialize AK002, its lead compound; the Company’s ability to obtain required regulatory approvals for its product candidates; uncertainties related to the enrollment of patients in its clinical trials; the Company’s ability to demonstrate sufficient safety and efficacy of its product candidates in its clinical trials; uncertainties related to the success of later-stage clinical trials, regardless of the outcomes of preclinical testing and early-stage trials; market acceptance of the Company’s product candidates; uncertainties related to the projections of the size of patient populations suffering from some of the diseases the Company is targeting; the Company’s ability to advance additional product candidates beyond AK002; the Company’s ability to obtain additional capital to finance its operations; and other risks described in the “Risk Factors” section included in our periodic filings that we have made and will make with the Securities and Exchange Commission (“SEC”). In addition, Allakos operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for Allakos’s management to predict all risks, nor can Allakos assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements that Allakos may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Allakos does not undertake any obligation to update or revise any forward-looking statements, to conform these statements to actual results or to make changes in Allakos’ expectations, except as required by law. Accuracy of Data: This presentation contains statistical data based on independent industry publications or other publicly available information, as well as other information based on Allakos’s internal sources. We have not independently verified the accuracy or completeness of the data contained in these industry publications and other publicly available information. Accordingly, Allakos makes no representations as to the accuracy or completeness of that data. Additional Information:The Company has filed and will file Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, and Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about the Company. You may get these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation concerns products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated.

Agenda Robert Alexander, PhD Introductions AK002 5:00 – 5:15 PM Henrik Rasmussen, MD PhD Review of Clinical Program 5:15 – 5:20 PM C. Stephen Foster, MD & Stephen Anesi, MD Overview of Ocular Allergy AK002 in Severe Allergic Conjunctivitis Phase 1b Study 5:20 – 6:00 PM Q&A 6:00 – 6:20 PM

Introduction Robert Alexander, PhD CEO – Allakos

Executive Summary Clinical stage company focused on the development of AK002, an anti-Siglec-8 mAb Lead indication is Eosinophilic Gastritis and/or Gastroenteritis Phase 2 study results expected July/August 2019 Recently reported AK002 clinical activity Rapid depletion of blood eosinophils in healthy volunteers and all studies to date Studies show symptom and quality of life improvements in multiple diseases including: Indolent Systemic Mastocytosis, Chronic Spontaneous Urticaria and two forms of Chronic Inducible Urticaria Today Positive data from AK002 Severe Allergic Conjunctivitis Phase 1 clinical study Significant improvements in comorbid atopic dermatitis, asthma, and rhinitis AK002 has the potential to be best-in-class in multiple mast cell and eosinophilic diseases

Mast Cells and Eosinophils: Effector Cells Central to Initiating and Maintaining Inflammatory Responses Found at the Internal/External Interface of the Body In particular, in tissues and surrounding blood vessels and peripheral nerves Produce a Broad Range of Inflammatory Mediators Vasoactive amines, lipid mediators, proteases, cytokines and chemokines Participate in Acute and Chronic Inflammation Including both innate and adaptive immune responses Key Drivers in Many Serious Diseases Including gastrointestinal, ophthalmic, dermatologic, respiratory, and proliferative diseases MAST CELLS EOSINOPHILS

AK002 Developed to Target Siglec-8 on Mast Cells and Eosinophils Cell Membrane Activating Receptors Activation Siglec-8 Mast Cell Eosinophil Inflammatory Response AK002 Inhibition Mast Cell Eosinophil Inhibition ADCC/Apoptosis AK002

Mast Cells and Eosinophils are Key Drivers of Atopic & Inflammatory Disease T Cell B Cell Activated B Cell Mast Cell IgE Activation and Recruitment of Other Immune Cells and Tissue Inflammation Macrophage Eosinophil Neutrophil Allergens Smooth Muscle Neuron Epithelium ACUTE AND CHRONIC INFLAMMATION SENSITIZATION Histamine, LTC4, PGD2 and proteases Bronchoconstriction, increased GI motility, pain, itch IL-33 IL- 4 IL-13 IL- 4 IL-13 Tissue damage, fibrosis ECP, MBP, elastase, MMP, TNFa , IL-1b, TGFb CCL5 IL-8 Histamine Substance P IL-6, TNFa

Eosinophils and Mast Cells Play a Significant Role in Many Diseases Mast Cell Activation Syndrome Indolent Systemic Mastocytosis Chronic Urticaria Atopic Dermatitis Eosinophilic Gastritis Eosinophilic Esophagitis Eosinophilic Gastroenteritis Ulcerative Colitis Vernal Keratoconjunctivitis GASTROINTESTINAL DISEASES OPHTHALMIC DISEASES RESPIRATORY DISEASES SKIN DISEASES MULTI-ORGAN DISEASES Atopic Keratoconjunctivitis Asthma Perennial Allergic Conjunctivitis Eosinophilic Asthma Idiopathic Pulmonary Fibrosis Irritable Bowel Syndrome Crohn’s Disease

AK002 Clinical Development Overview Henrik Rasmussen, MD PhD CMO – Allakos

AK002 Preclinical Phase 1 Phase 2 Data Expected Eosinophilic Gastritis July/August 2019 Chronic Urticaria Presented Q1 2019 Indolent Systemic Mastocytosis Presented Q1 2019 Severe Allergic Conjunctivitis Presented Today Current AK002 Development Status

AK002 Clinical Data to Date Key Findings Healthy Volunteers Rapid depletion of eosinophils Dose-dependent duration of eosinophil depletion Chronic Urticaria High response rates in multiple forms of antihistamine-resistant chronic urticaria, including omalizumab-refractory and inducible urticaria Indolent Systemic Mastocytosis Significant symptom and quality of life improvement

AK002 Safety Summary No adverse findings in short- and long-term animal toxicity studies PRECLINICAL Approximately 180 subjects exposed to drug in clinical studies Generally well-tolerated Mild to moderate infusion reactions (flushing, feeling of warmth, headache, nausea, or dizziness) consistent with other mAbs with ADCC activity ~19% IRR rate on first infusion ~2% IRR rate on subsequent infusions CLINICAL

Severe Allergic Conjunctival Disease Phase 1b Study Design Key Endpoints Status Open-label, pilot study 30 patients – 3 cohorts Atopic keratoconjunctivitis Vernal keratoconjunctivitis Perennial allergic conjunctivitis Dosed once monthly for 6 months 0.3 mg/kg starting dose, followed by 1.0 mg/kg then either 1.0 mg/kg or 3.0 mg/kg, based on symptoms Primary Safety and tolerability 29 patients 13 AKC 15 PAC 1 VKC Topline data presented today Secondary Allergic Conjunctivitis Symptom (ACS) PRO: Itching, photophobia, foreign body sensation, ocular pain, and lacrimation Ocular Symptom Score (OSS) Investigator assessment: Itching, redness, tearing, and chemosis Atopic comorbidities assessment: Atopic dermatitis Asthma Rhinitis

C. Stephen Foster, MD and Stephen Anesi, MD C. Stephen Foster, MD - Principal Investigator Founder and president of MERSI Professor, Ophthalmology & Adjunct Professor, Allergy & Immunology, Harvard Developed standard of care in treating uveitis/ocular inflammatory diseases Currently serves on >10 committees, including International Society of Ocular Pharmacology and Pharmaceutics and the International Uveitis Study Group >20 editorial board positions >800 peer reviewed publications, >100 books and chapters Stephen Anesi, MD - Sub-Investigator Associate partner at MERSI Completed fellowship in Ocular Immunology and Uveitis under Dr. Foster >15 peer reviewed publications, >5 books and book chapters

Phase 1b: AK002 in Patients with Severe Allergic Conjunctivitis C. Stephen Foster, MD Stephen Anesi, MD

AKC, PAC, & VKC are Severe Forms of Allergic Conjunctivitis CLINICAL FEATURES IMPACT Symptoms Extreme Itching, Photophobia, Pain, Sensation of Foreign Body, Burning, Watering, Mucous Discharge Poor Quality of Life Signs Redness (Hyperemia), Swelling (Chemosis, Periorbital Edema), Tarsal Papillae, Cicatricial Changes, Corneal Damage (Keratitis, Epithelial Erosion, Ulcers) Vision Loss, Poor Quality of Life Atopic Comorbidities Common Atopic Comorbidities Include Atopic Dermatitis, Asthma, and Rhinitis High Systemic Disease Burden, Poor Quality of Life

Severe Allergic Conjunctivitis Giant Papillae Corneal Ulcer (Vision Loss) Redness, Chemosis Photophobia, Watering, Periorbital Swelling

Perennial Allergic Conjunctivitis (PAC) Vernal Keratoconjunctivitis (VKC) Atopic Keratoconjunctivitis (AKC) Mast Cells +++ +++ ++ Eosinophils ++ ++ +++ T and B cells + ++ +++ Fibroblasts ++ ++ Immune Cells Involved in Allergic Conjunctivitis Mast cells and eosinophils are key effector cells in allergic conjunctivitis Source: Leonardi A. “Immunopathogenesis of ocular allergy: a schematic approach to different clinical entities.” Curr Opin in Allergy Clinical Immunol. 2007, 7:429-435; Tsubota K. “Detection by brush cytology of mast cells and eosinophils in allergic and vernal conjunctivitis.” Cornea. 1991;10(6):525.

Limitations of Current Treatment Options Antihistamines / Mast Cell Stabilizers Require frequent dosing (up to 4 times per day) Typically ineffective in severe cases Ocular Topical Steroids Associated with multiple adverse effects, including cataracts and glaucoma Chronic, long-term use significantly increases risk of irreversible vision loss Require frequent monitoring of intraocular pressure (every 1 to 5 weeks) Calcineurin Inhibitors Limited efficacy and safety data Access and reimbursement often challenging due to lack of labeled indication Source: Leonardi et al. Allergy. 2019; Phulke et al. J Curr Glaucoma Pract. 2017; La Rosa et al. Ital J Pediatr. 2013; Weiner, American Academy of Ophthalmology EyeNet. Feb 2013

US Prevalence of Severe Allergic Conjunctivitis US population experiencing Ocular Allergy Symptoms ~130 Million1 ~7.4 Million2 Diagnosed Allergic Conjunctivitis patients ~6% visit a physician for their condition (1) 40% of US population, Singh et al. J Allergy Clin Immunol. 2010 and Bielory et al. Allergy Asthma Proc. 2014; (2) Allakos analysis of Symphony Health PatientSource claims; (3) chronic use defined as 2 or more prescriptions for ocular steroids per year for 2 or more consecutive years ~120,0002 Severe Allergic Conjunctivitis patients on chronic ocular steroids3 ~2% receive chronic ocular steroids

Severe Allergic Conjunctivitis – Unmet Need ~120,000 patients in the US have antihistamine-refractory severe allergic conjunctivitis Significant disease burden, which can lead to blindness There are no safe chronic treatment options available

Severe Allergic Conjunctival Disease Phase 1b Study Design Key Endpoints Status Open-label, pilot study 30 patients – 3 cohorts Atopic keratoconjunctivitis Vernal keratoconjunctivitis Perennial allergic conjunctivitis Dosed once monthly for 6 months 0.3 mg/kg starting dose, followed by 1.0 mg/kg then either 1.0 mg/kg or 3.0 mg/kg, based on symptoms Primary Safety and tolerability 29 patients 13 AKC 15 PAC 1 VKC Topline data presented today Secondary Allergic Conjunctivitis Symptom (ACS) PRO: Itching, photophobia, foreign body sensation, ocular pain, and lacrimation Ocular Symptom Score (OSS) Investigator assessment: Itching, redness, tearing, and chemosis Atopic comorbidities assessment: Atopic dermatitis Asthma Rhinitis

Baseline Characteristics (1)By medical history AKC (N=13) VKC (N=1) PAC (N=16) Total (N=30) Age, Median (Range) 50 (23-72) 25 55 (29-79) 52 (23-79) Female 38% 0 63% 50% Age of AC Onset, Median (Range) 36 (7-72) 12 46 (19-69) 43 (7-72) Years with AC, Median (Range) 6 (0-38) 13 4 (0-19) 6 (0-38) Atopic Comorbidities1 ≥1 Comorbidity 85% 100% 88% 87% ≥2 Comorbidities 69% 100% 44% 57% Atopic Dermatitis 85% 0 44% 60% Asthma 54% 100% 25% 40% Rhinitis 54% 100% 75% 67%
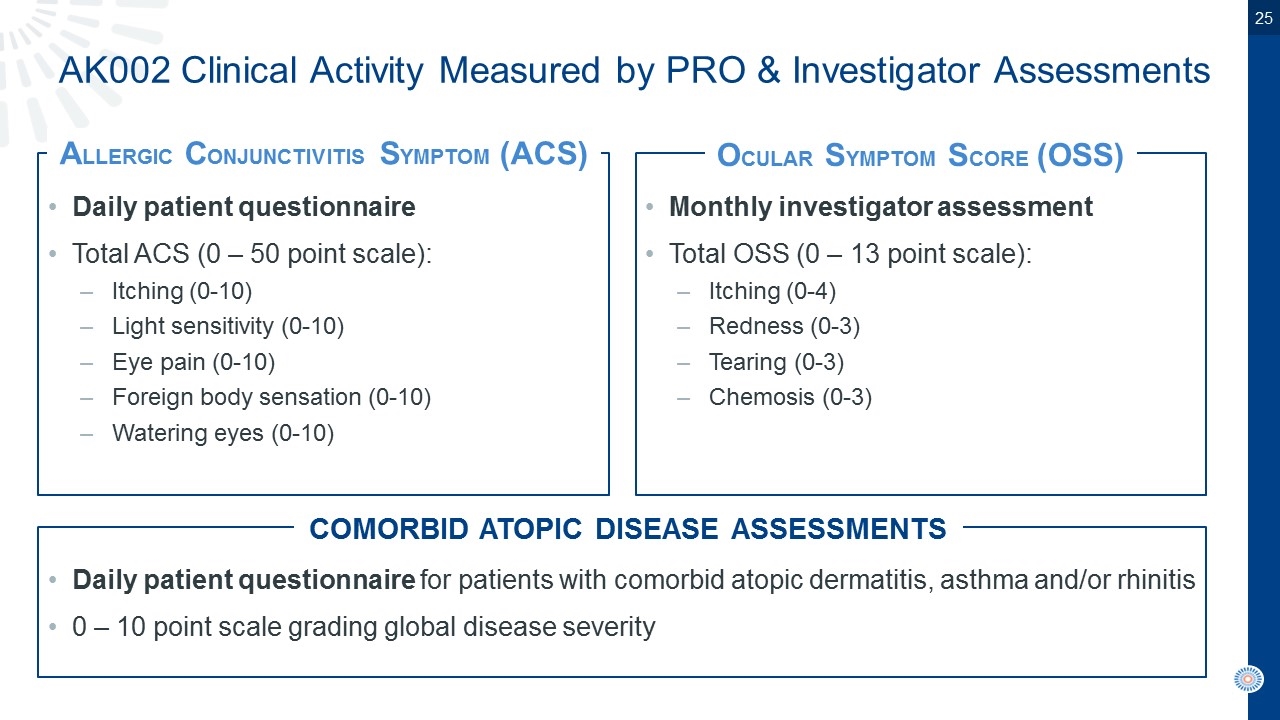
AK002 Clinical Activity Measured by PRO & Investigator Assessments Daily patient questionnaire Total ACS (0 – 50 point scale): Itching (0-10) Light sensitivity (0-10) Eye pain (0-10) Foreign body sensation (0-10) Watering eyes (0-10) Monthly investigator assessment Total OSS (0 – 13 point scale): Itching (0-4) Redness (0-3) Tearing (0-3) Chemosis (0-3) Daily patient questionnaire for patients with comorbid atopic dermatitis, asthma and/or rhinitis 0 – 10 point scale grading global disease severity ALLERGIC CONJUNCTIVITIS SYMPTOM (ACS) OCULAR SYMPTOM SCORE (OSS) COMORBID ATOPIC DISEASE ASSESSMENTS

Improvements in Allergic Conjunctivitis Signs & Symptoms Median Total ACS Baseline Weeks 21-22 Median Total OSS Baseline Day 140 -71% -78% Patient Reported Investigator Assessment All Subjects (n=29)

Substantial Improvements in Multiple Forms of Severe AC Median Total ACS AKC (n=13) Baseline Weeks 21-22 VKC (n=1) PAC (n=15) All Subjects (n=29) Baseline Weeks 21-22 Baseline Weeks 21-22 Baseline Weeks 21-22 -74% -87% -71% -78%

Consistent Improvements Across Signs & Symptoms Symptom Median % Δ from BL to Wk 21-22 Allergic Conjunctivitis Symptom (ACS) Patient Reported - Daily Itching -75% Light Sensitivity -57% Eye Pain -75% Foreign Body Sensation -80% Watering Eyes -76% Symptoms & Signs Median % Δ from BL to Day 140 Ocular Symptom Score (OSS) Investigator Assessment - Monthly Itching -67% Redness -67% Tearing -50% Chemosis -100%

Substantial Improvement in Atopic Comorbidities Atopic Dermatitis (n=11) Baseline Weeks 21-22 -65% Asthma (n=9) Baseline Weeks 21-22 Rhinitis (n=11) Baseline Weeks 21-22 -72% -69% Median Symptom Score

Severe Allergic Conjunctivitis Phase 1b: Safety Summary Generally very well-tolerated No drug-related Serious Adverse Events Most common adverse event was mild to moderate infusion-related reactions (IRRs; flushing, feeling of warmth, headache, nausea, or dizziness) 16.7% IRRs rate on first infusion 0.7% IRRs rate on subsequent infusions

Patient Case Studies C. Stephen Foster, MD Stephen Anesi, MD

Case Study 1: AKC with Comorbid Atopic Dermatitis & Rhinitis Medical History 27 year-old male with severe AKC, atopic dermatitis, and rhinitis Baseline normal peripheral blood eosinophils (190 eos/µL) Suffered from severe symptoms despite treatment Itching, foreign body sensation, and watering Hyperemia (redness) and palpebral papillae Moderate comorbid atopic dermatitis & rhinitis Treatment history AKC: topical antihistamines, topical corticosteroids Atopic Dermatitis: oral antihistamines Rhinitis: oral antihistamines

Case Study 1: Improvement in Ocular Symptoms 99% reduction in Total ACS Watering Foreign Body Eye Pain Light Sensitivity Itching Total ACS Month 1 Month 2 Month 3 Month 4 Month 5 Weeks 21-22

Case Study 1: Improvement in Ocular Signs and Symptoms and Comorbid Atopic Dermatitis and Rhinitis Total ACS Baseline Weeks 21-22 Total OSS -100% Baseline Day 140 Atopic Dermatitis Baseline Weeks 21-22 Rhinitis Baseline Weeks 21-22 -99% -82% -100% Symptom Score

Case Study 1: Reversal of Neovascular and Inflammatory Changes Prior to AK002 After 3 Doses of AK002

Case Study 2: AKC with Comorbid Atopic Dermatitis & Asthma Medical History 49 year-old male with severe AKC, atopic dermatitis, and asthma Baseline high peripheral blood eosinophils (1350 eos/µL) Suffered from severe symptoms despite treatment Photophobia, conjunctival hyperemia, and chemosis Periorbital atopic dermatitis and edema Moderate-to-severe comorbid asthma Treatment history AKC: topical corticosteroids, topical antihistamines, topical cromolyn Atopic Dermatitis: dupilumab, topical corticosteroids, topical tacrolimus Asthma: daily ICS/LABA, cromolyn, albuterol

Case Study 2: Improvement in Ocular Symptoms 97% reduction in Total ACS Watering Foreign Body Eye Pain Light Sensitivity Itching Total ACS Month 1 Month 2 Month 3 Month 4 Month 5 Weeks 21-22

Case Study 2: Improvement in Ocular Signs and Symptoms and Comorbid Atopic Dermatitis and Asthma Total ACS Baseline Weeks 21-22 Total OSS -82% Baseline Day 140 Atopic Dermatitis Baseline Weeks 21-22 Asthma Baseline Weeks 21-22 -97% -36% -78% Symptom Score

Case Study 2: Substantial Clinical Improvements Prior to AK002 After 3 Doses of AK002

Case Study 3: AKC with Comorbid Atopic Dermatitis, Asthma, and Rhinitis Medical History 53 year-old male with AKC, atopic dermatitis, asthma, and rhinitis Baseline normal peripheral blood eosinophils (120 eos/µL) Suffered from severe symptoms despite treatment Itching, photophobia, foreign body sensation, redness, & chemosis Severe comorbid atopic dermatitis & asthma Treatment history AKC: topical corticosteroids, topical antihistamines Atopic Dermatitis: dupilumab, topical corticosteroids Asthma: daily ICS/LABA, albuterol Rhinitis: oral antihistamines

Case Study 3: Improvements in Ocular Signs and Symptoms and Comorbid Atopic Dermatitis, Asthma, and Rhinitis Total ACS Baseline Weeks 21-22 Atopic Dermatitis -67% Baseline Weeks 21-22 Asthma Baseline Weeks 21-22 Rhinitis Baseline Weeks 21-22 -88% -82% -96% Symptom Score

Case Study 4: AKC w/ Comorbid Esophagitis, Gastritis, Duodenitis, Urticaria Medical History 45 year old female with severe AKC, GI disease, and chronic urticaria Baseline normal peripheral blood eosinophils (160 eos/µL) Suffered from severe symptoms despite treatment Itching, photophobia, discomfort, and watering Frequent and severe stomach pain, nausea, and diarrhea Frequent and severe headaches / migraines Frequent spontaneous and inducible urticaria (edema, hives, rash, and flushing) Treatment history AKC: oral corticosteroids, topical cromolyn, oral antihistamines Gastrointestinal Diseases: restricted diet, PPIs, oral cromolyn sodium Urticaria: oral antihistamines, lifestyle modification / trigger avoidance

Case Study 4: Improvement in Ocular Symptoms Total OSS Watering Foreign Body Eye Pain Light Sensitivity Itching Total ACS 99% reduction in Total ACS 100% reduction in Total OSS Month 1 Month 2 Month 3 Month 4 Month 5 Weeks 21-22

Case Study 4: Improvements in Ocular Signs and Symptoms Total ACS Baseline Weeks 21-22 Total OSS Baseline Day 140 -100% -99% Patient Reported Investigator Assessment Case Study 4: AKC Patient

Summary AK002 demonstrated clinical activity in severe allergic conjunctivitis AK002 is a targeted therapy that may represent a novel alternative to chronic steroid use Results suggest significant activity in systemic atopic comorbidities such as atopic dermatitis, asthma, and rhinitis

Executive Summary Clinical stage company focused on the development of AK002, an anti-Siglec-8 mAb Lead indication is Eosinophilic Gastritis and/or Gastroenteritis Phase 2 study results expected July/August 2019 Recently reported AK002 clinical activity Rapid depletion of blood eosinophils in healthy volunteers and all studies to date Studies show symptom and quality of life improvements in multiple diseases including: Indolent Systemic Mastocytosis, Chronic Spontaneous Urticaria and two forms of Chronic Inducible Urticaria Today Positive data from AK002 Severe Allergic Conjunctivitis Phase 1 clinical study Significant improvements in comorbid atopic dermatitis, asthma, and rhinitis AK002 has the potential to be best-in-class in multiple mast cell and eosinophilic diseases

Q&A

Thank you
